Should CRISPR Scientists Play God?
Will genome editing with the new technology, CRISPR, usher in a new era of Promethean overreach? CRISPR makes altering the human genome widely available and cheap. But the fur is rising on the necks of anti-play-god bioethicists; they fear that geneticists will play god and precipitate a backlash from nature that could be disastrous to the human race. In contrast to the anti-play-god bioethicists, Ted Peters’ newly published article, “Should CRISPR Scientists Play God?”, recommends that laboratory science invoke the Precautionary Principle (PP). With the PP in hand, laboratory researchers should pause at the yellow caution light. But then, with constant risk-assessment, proceed ahead.
Here is what you might want to know but really don’t need to. CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. What does this mean? In our evolutionary past, our human genomes incorporated palindromic DNA repeats from bacteria and archaea which are their adaptive method for strengthening their immune systems. The summary point to get is this: palindromic repeats of DNA base pairs provide targets for the geneticist to shoot at.
The CRISPR archer shoots at these targets with Cas9 arrows. What’s Cas9? It’s an endonuclease capable of cleaving DNA. When combined with specific RNA in a system it can either insert or delete specific genetic sequences. If Cas9 is the arrow, the CRISPR archer can fire it to a specific target on a DNA strand, cut it, insert a prescribed sequence of nucleotides, and then re-connect the DNA strand. We call this “gene editing” for short.
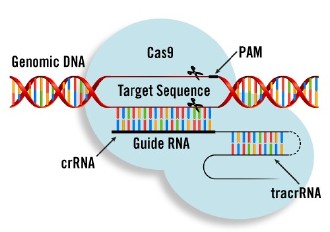
Here is the upshot. CRISPR/Cas9 technology can be used for highly specific and convenient gene editing, either inserting sequences in target genes, deleting genes, or turning genes off. The overwhelming scientific consensus is that this technology will usher in an age of cheap and easy genetic manipulation. If we don’t like the DNA nature has bequeathed us, we can employ CRISPR/Cas9 to edit it to our standards.
There are two important ways that CRISPR/Cas9 technology can improve human health and wellbeing. The first is somatic genome editing therapy. A patient suffering from metastatic non-small lung cancer, for example, may benefit. How might this work? We note that everyday our immune system engages cancer threats with a defense. When the defense is compromised, cancer wins. On the front line is the T cell which, like Achilles, leads the immune system into battle against cancer. This happens commonly. Right now while you’re reading this text the battle between your immune system and cancer is taking place. In persons with metastatic non-small lung cancer for whom chemotherapy and radiation have failed, however, scientists have observed that T cells are sabotaged from within by a Quisling enzyme, PD-1. By sending CRISPR/Cas9 into these T cells, clinicians are hoping to snip out the PD-1 gene and liberate the T cell. The T cell should then triumph once again in the cancer siege. By editing the genome of a living patient, this type of cancer might succumb to somatic genome editing therapy.
The second potential way in which CRISPR/Cas9 editing might contribute to human health is through germline editing. Our germ cells are found in eggs and sperm, where they are in a position to pass our DNA on through our children to many future generations. On the tip of chromosome 4, for another example, some sufferers from Huntington’s disease have a mutated gene (allele) which is responsible for untold suffering and crippling. Should a CRISPR scientist edit this gene in the germline, thereby eliminating it for this person’s descendents? Should our generation systematically eliminate this allele from the human gene pool, thereby eliminating this disease for everyone in the future?
“Whoah!” shouts the anti-play-god bioethicist. Why? Are the bioethicists heartless? Do bioethicists want to see Huntington’s patients suffer? No, that is not the reason. Their judgment is based on what we don’t know. What we don’t know is the long-term effect of such large-scale changes in the genome. Genes work with other genes and other DNA in delicate systems like Swiss watches, mutually influencing one another. To eliminate one set of gears in an old fashioned Swiss watch would cause it to self-destruct. Might this analogy apply to the human genome? We don’t know yet. Without this knowledge, clinical geneticists cannot measure the risk.
Without knowing the level of harm-benefit risk, the question arising among bioethicists is this: should we proceed to modify the germline in human beings (and plants or animals too)? On most days when such questions arise, the average bioethicist can get by with a single-word vocabulary. All he or she needs to do is pronounce the word “No,” with emphasis, and the job is done. If a bioethicist were to say, “Yes” too often, he or she would be shunned by colleagues as having sold out to the industry.
The PP to the rescue. Peters relies on the so-called Wingspread version of the Precautionary Principle as it was formulated at the 1992 United Nations Conference on Environment and Development: “When an activity raises threats of harm to human health or the environment, precautionary measures should be taken even if some cause and effect relationships are not fully established scientifically. In this context the proponent of the process or product, rather than the public, should bear the burden of proof.” Although the PP applied originally to ecological ethics, it might apply equally well to gene editing.
Here’s what I recommend: “Yes, but not yet.” That’s a longer sentence than merely the word, “No”. “Yes, but not yet” might be the most appropriate ethical counsel we could offer to those geneticists attempting to ascertain the risk level of CRISPR editing of the human germline.
CRISPR/Cas9 puts us momentarily at a traffic stoplight. We have three options. Those who affirm Prometheus’ hubris and invoke the technological imperative—if we can do it we should do it!—want to race through a green light toward an enhanced humanity. The anti-play-god Promethean hopes that the light will remain red so all traffic remains stopped. The proceed-with-caution bioethicist looks both ways on yellow, but drives forward. Peters recommends the third.
|
*For you who do not regularly read my blog, I am a fictional character in the thriller,
For God and Country. I am a former CIA operative currently serving as a Lutheran
pastor on the south side of Chicago. My doctorate from Michigan State University is in astrobiology and I give special attention to Society, Science, and Spirit.
Genetic Engineering Will Change Everything Forever – CRISPR
16 Minute illustrated history & tutorial ★★★★
The Gene Editors Are Only Getting Started
Would you eradicate malaria-carrying insects? Change your baby’s DNA? Scientists soon may have the power to do both.
Just what is CRISPR? Simply put a method of gene editing. Rewriting the code of life has never been so easy. In 2012 scientists demonstrated a new DNA-editing technique called Crispr. Five years later it is being used to cure mice with HIV and
hemophilia. Geneticists are engineering pigs to make them suitable as human organ donors. Bill Gates is spending $75 million to endow a few Anopheles mosquitoes, which spread malaria, with a sort of genetic time bomb that could wipe out the species. A team at Harvard plans to edit 1.5 million letters of elephant DNA to resurrect the woolly mammoth.
“I frankly have been flabbergasted at the pace of the field,” says Jennifer Doudna, a Crispr pioneer who runs a lab at the University of California, Berkeley. “We’re barely five years out, and it’s already in early clinical trials for cancer. It’s unbelievable.”
The thing to understand about Crispr isn’t its acronym—for the record, it stands for Clustered Regularly Interspaced Short Palindromic Repeats—but that it makes editing DNA easy, cheap and precise. Scientists have fiddled with genes for decades, but in clumsy ways. They zapped plants with radiation to flip letters of DNA at random, then looked for useful mutations. They hijacked the infection mechanisms of viruses and bacteria to deliver beneficial payloads. They shot cells with “gene guns,” which are pretty much what they sound like. The first one, invented in the 1980s, was an air pistol modified to fire particles coated with genetic material.
Crispr is much more precise, as Ms. Doudna explains in her new book, “A Crack in Creation.” It works like this: An enzyme called Cas9 can be programmed to latch onto any 20-letter sequence of DNA. Once there, the enzyme cuts the double helix, splitting the DNA strand in two. Scientists supply a snippet of genetic material they want to insert, making sure its ends match up with the cut strands. When the cell’s repair mechanism kicks in to fix the cut, it pastes in the new DNA.
It’s so exact that Crispr blurs the meaning of “genetically modified organism.” The activists yelling about “frankenfish” are generally upset about transgenic plants and animals—those with DNA inserted from other species. But what about using Crispr to alter only a few letters of an organism’s own genome, the kind of mutation that could happen naturally?
Last year a professor at Penn State created blemish-resistant mushrooms by knocking out a gene that causes them to turn brown when handled. “It attracted attention,” Ms. Doudna says, “because the U.S. Department of Agriculture ruled that that type of plant product would not be regulated as a genetically modified organism.”
Ms. Doudna welcomes this kind of streamlining as the Food and Drug Administration considers its own approach to Crispr crops. “It’s crazy. It takes years and years and years to bring a plant to market,” she says. “I’m all for safety of course and that has to come first. But I think it has to be done with knowledge of the science that makes sense.”
Medical labs are also putting Crispr to work, since it is potentially meticulous enough for routine use on people. The human genome is 3.2 billion letters, and in the wrong place a single typo—a dozen or so misplaced atoms—can create misery. For patients with disorders like cystic fibrosis, the obstacles to fixing the genetic glitch with Crispr seem mostly practical.
First, there’s delivery: A human body contains some 50 trillion cells. How do you get Crispr to the affected ones, and what percentage need to be edited successfully to matter? Ms. Doudna says injecting Crispr-laden viruses into animal tissues has resulted in rates of editing on the order of 70%—enough to have a therapeutic benefit: “In muscular dystrophy, for example, it looks like you only need to have somewhere between 10% to 20%.”
Second, there’s the risk: Although Crispr aims at a 20-letter DNA sequence, occasionally it can hit a partial match and make an unintended edit. “For any drug that we’re developing for treatment, you’re going to have some kind of risk factors,” Ms. Doudna says. “In this case it might be changes to DNA, and you have to decide what’s the right level that you would tolerate.” There are ways to minimize the mistakes, and some studies show so few off-target edits “that it’s difficult to distinguish them from just errors in DNA sequencing.”
What seems to merit the risk today? “Sickle-cell disease,” Ms. Doudna says: “Well-known mutation. Single gene is involved. No treatments right now for people. They have these horrible crises where they’re in terrible pain.” Moreover, the faulty red blood cells can be drawn from a vein and isolated. “The actual DNA editing can be done outside the body,” she says, “validated first, and then the cells implanted and allowed to repopulate the blood supply.” The approach may work for cancer, too: A Crispr clinical trial awaiting FDA approval would pull white blood cells, give them tumor-killing superpowers, and then put them back into action.
It would be technically simpler, rather than working in fully grown patients, to fix genetic disorders early, in human eggs, sperm or embryos. But this raises thorny moral questions, since edits made to these cells would pass down to future generations, who can’t consent to having their genes tweaked. In debates about this, the word “eugenics” comes up.
At first, Ms. Doudna was reflexively opposed. “I’m not a religious person,” she says, “but it’s more, just—I don’t know—sort of an intrinsic reaction, that it feels like a realm where maybe we shouldn’t be messing around.” Her position softened, somewhat to her own surprise, as she heard from hundreds of people facing horrific genetic diseases. “They’re reaching out because they’re desperate,” she says. “A lot of them are asking me the questions you’re asking about: How soon? How long will it be? Is there hope for my child?”
Ms. Doudna recalls an email from a 26-year-old woman who’d found out she carried a mutation in the gene BRCA1 that is associated with a 60% risk of breast cancer by age 70: “She said, ‘Should I have a mastectomy?’ ”—this was right after Angelina Jolie, worried about a similar mutation, did the same—“ ‘Or do you think that gene-editing is going to come along in time for me? Or if not for me, at least so that I can get rid of this mutation in my eggs?’ ”
There was a man who watched his father die of Huntington’s disease and had three sisters diagnosed. There was a woman whose daughter had given birth to a child with Fragile X syndrome, which causes intellectual disability, but deeply wanted to conceive again. “She was very emotional,” Ms. Doudna recounts. “She said, ‘If there were a way to use this, and if I could use it in embryos or germ cells, I would have absolutely no hesitation about doing it.’ ”
A few bioethicists have even argued that research on editing human embryos is a “moral imperative,” since roughly 6% of all babies have “serious birth defects.” As for the risk of “off target” edits, merely smoking cigarettes can create mutations in a man’s sperm. One academic joked that if old-fashioned sex were up for regulatory review, the FDA would never sign off.
Not everyone has the same reaction. A reporter interviewing Ms. Doudna once revealed she had a son with Down syndrome. “She said, ‘I just want you to know that he’s perfect just the way he is.’ It was very touching,” Ms. Doudna recalls, her voice flickering with emotion. Even if Crispr could have fixed that genetic defect, the woman said she wouldn’t change it. Some people in the deaf community feel the same way, and Ms. Doudna respects that. “Everyone’s feeling about DNA and about their inheritance and their children is going to be different,” she says. “It has to be a choice. People can decide what they want to do.”
Ms. Doudna remains opposed to nontherapeutic editing, often characterized as “designer babies,” and she says regulators won’t allow it, at least in the U.S. But other countries are less stringent. Is news of the first Crispr baby simply going to break one day? “It would be naive to think that that won’t happen at some point,” she says. Pressure to push forward will come not only from desperate people but also clinics abroad that may drum up business by saying: “We’ll do things here that will be advantageous for your children that are not allowed elsewhere.”
That’s why Ms. Doudna sees the ethical debate as vital. “It’s very hard to enforce any kind of global regulations on anything, but certainly on science,” she says. “So I think the next best thing is to try to encourage a global consensus that is strong enough that people feel some pressure to conform to it.”
The plan to eradicate the Anopheles mosquito presents a similar problem of collective decision-making. One iteration would involve a version of the insect edited to carry DNA that creates sterile females. That trait could then be forced into the wild population using a “gene drive.” Recall the basic rules of heredity—think back to that Punnett square from high school. Normally, an edited male in the wild would pass on the sterility gene to only half its offspring. Over many generations, the edited DNA would be diluted into oblivion.
That’s where the gene drive comes in. Scientists using Crispr in the lab have given the mosquito DNA that causes its cells to create Crispr. The result is a recursive, self-propagating gene that slices its reproductive competition. The edited mosquito passes on the sterility gene to nearly 100% of its offspring—which in turn do the same. Theoretically, releasing a single gene-drive insect, or letting one escape out an air-conditioning vent, could spread the edited DNA to the entire species.
Theoretically. “Although we understand that these gene drives can work in a laboratory setting efficiently in fruit flies and things like that, how well would they really work environmentally?” Ms. Doudna asks. “Evolution is a very strong force. If you put a species in a wild setting where they have to compete with other species, if they have a disadvantage reproductively, even if it’s a small disadvantage, they’re going to lose out.”
Ms. Doudna still needs to be convinced, too, of the wisdom of letting loose a gene drive. She cites her native Hawaii. “Species were introduced to that environment that ended up having large unintended consequences,” she says. Seeing that made her “very respectful of nature and very cautious about human beings’ thinking they have the knowledge to predict what will happen.”
A final Crispr worry is that it makes DNA editing so easy anybody can do it. Simple hobby kits sell online for $150, and a community biotech lab in Brooklyn offers a class for $400. Jennifer Lopez is reportedly working on a TV drama called “C.R.I.S.P.R.” that, according to the Hollywood Reporter, “explores the next generation of terror: DNA hacking.”
Ms. Doudna provides a bit of assurance. “Genetics is complicated. You have to have quite a bit of knowledge, I think, to be able to do anything that’s truly dangerous,” she says. “There’s been a little bit of hype, in my opinion, about DIY kits and are we going to have rogue scientists—or even nonscientists—randomly doing crazy stuff. I think that’s not too likely.”
Still, a couple of years ago Ms. Doudna had a dream in which a colleague asked her to explain gene-editing to someone very important. Turns out it was Hitler, except with the face of a pig. This, she says now, was her awakening to Crispr’s potential. “Try to imagine: We’re biochemists here, we’re futzing around with bacteria, just fartin’ around the lab, and students are doing experiments,” she says. “Then suddenly you have this discovery that you realize can be harnessed in a very different way.”
A few moments later she adds: “It was just this growing realization that this is no joke. This is a really seriously powerful technology.”
Mr. Peterson is the Journal’s deputy editorial features editor.
What you need to know about CRISPR | Ellen Jorgensen a TED TALK VIDEO
